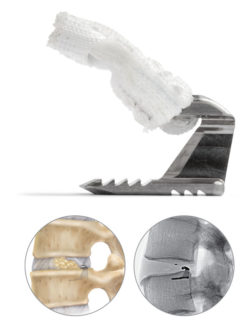
Intrinsic Therapeutics is the manufacturer of Barricaid®. Designed to prevent reherniation.
Adjacent to a lumbar discectomy, the Barricaid® device is designed to close large defects in the annulus, which often lead to disc reherniation and/or disc collapse. Barricaid is a permanent implant with two components – a titanium bone anchor and a flexible polymer barrier. The barrier is designed to prevent reherniation by physically blocking the annulus at the post-surgery defect. The anchor component is comprised of titanium alloy that is placed into either the caudal or cranial-adjacent vertebral body to secure the device in position.
Barricaid is a patented technology.