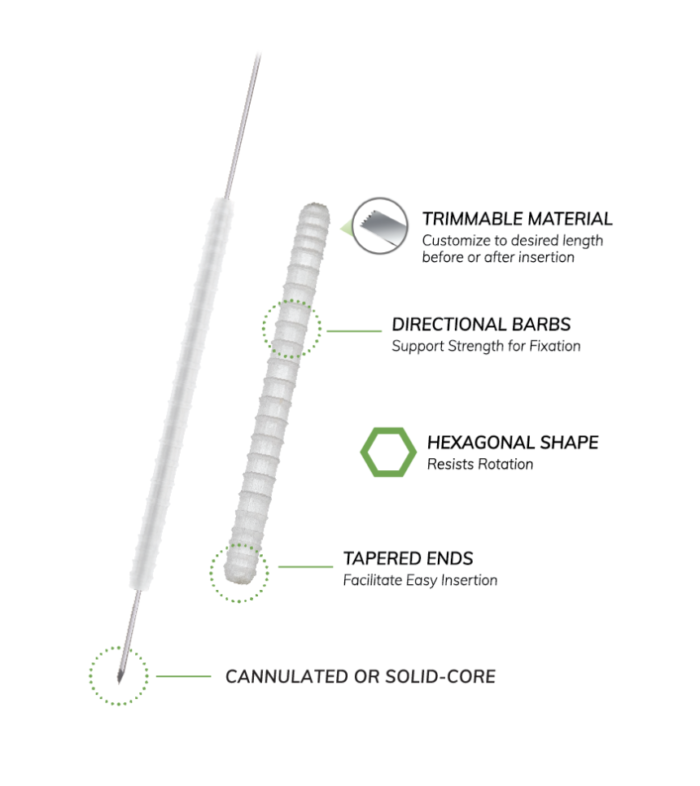
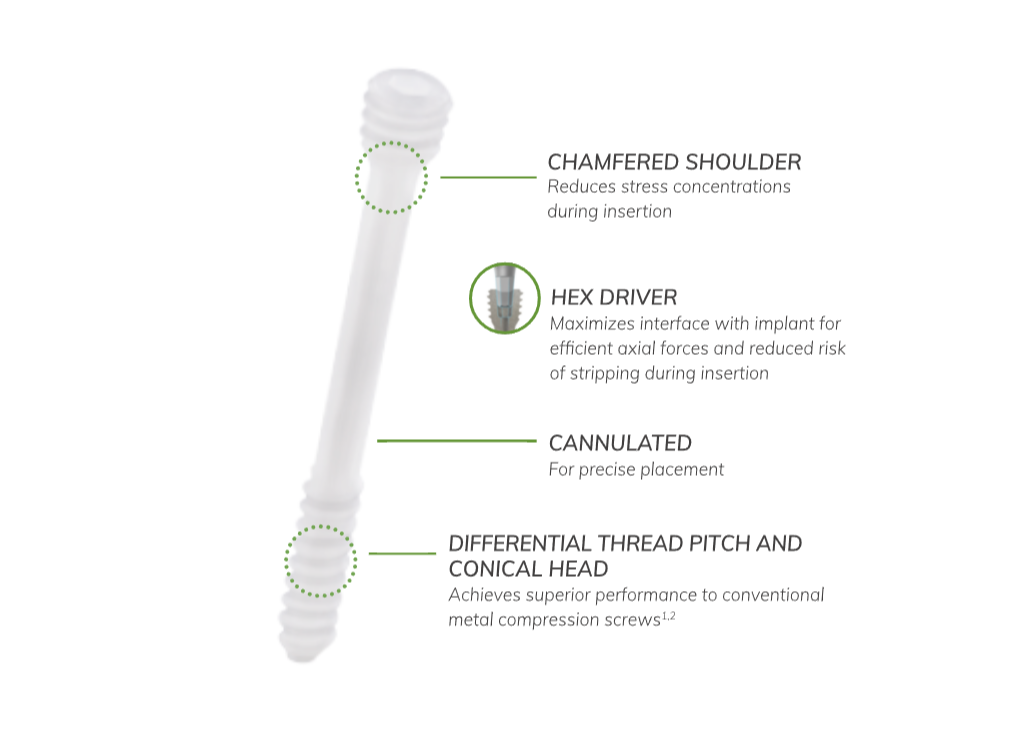
OSSIOfiber® is the first-of-its kind implant material to securely fixate and fully integrate into the native anatomy with nothing left behind.
Unlike other implants, OSSIOfiber® contains the same organic minerals found in bones themselves, but it’s stronger—it eventually becomes part of the bone, encouraging a return to full strength naturally without the risks and costs associated with permanent hardware.1