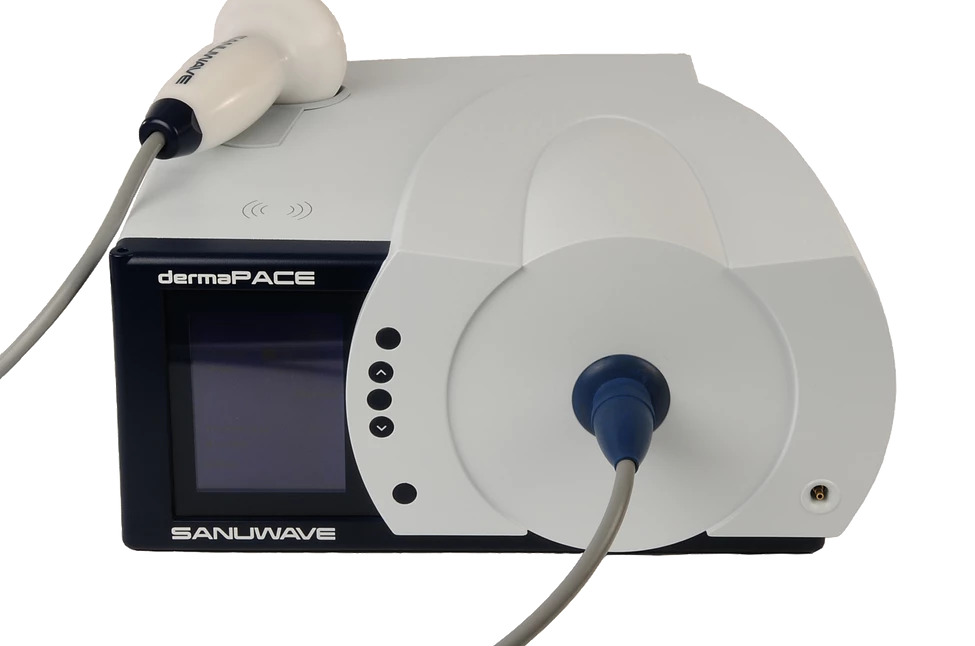
SANUWAVE is a shockwave technology company developing patented, noninvasive devices for the repair and regeneration of skin, musculoskeletal tissue and vascular structures. Its lead product, the dermaPACE System, is FDA Cleared for the treatment of Diabetic Foot Ulcers (DFU) in the United States.
For International Markets, the dermaPACE System is CE Marked for use on acute and chronic defects of the skin and subcutaneous soft tissue.